What is Cancer?
Cancer is the uncontrolled growth of cells that disrupts normal bodily functions. Successful treatments enable many individuals to lead fulfilling lives after cancer. It encompasses various types originating in different organs, such as the lungs, breast, colon, or blood. While cancers share similarities, their growth and spread differ. Normal cells divide orderly, but cancer cells multiply uncontrollably, crowding out healthy cells and causing complications. Common treatments include surgery, chemotherapy, and radiation.

What are Clinical Trials?
Clinical trials or research studies for cancer utilize patient volunteers to help investigate different ways to treat or manage cancer. Clinical trials involve the use of investigational or study therapies and their delivery methods. Each study tries to answer specific scientific questions about different ways to prevent, diagnose, treat, or manage the type of cancer the investigational therapy is testing.
Preparing for Your Appointment
We look forward to meeting you on your first visit at Mary Crowley Cancer Research. To assist in your visit preparation, we have provided information that will help acquaint you with our program and hopefully make it easier for you and your family.
You have the opportunity below, to print, complete and bring with you necessary forms for your initial consultation visit. We have also prepared a step-by-step document to guide you through your cancer journey at Mary Crowley. If your cancer molecular profile is not yet completed, learn about its importance in matching you with the most suitable clinical trial.
- Authorization for Release of Health Information
- Medication List Form
- Referring and Primary Care Physician Information Sheet
- Patient Registration Form – Part 1
- Patient Registration Form – Part 2
- Clinical Trials and Insurance Coverage – Understanding Out of Pocket Cost for Clinical Trial Participation
- Notification & Acknowledgement of Patient Financial Responsibilities
- Advance Care Planning and Directives Questionnaire
- Daily Activity Questionnaire
At Mary Crowley Cancer Research, we believe that your health information is personal. We keep records of the care and
services that you receive at our facilities. We are committed to keeping your health information private, and we are
also required by law to respect your confidentiality.
Please click below for a copy of the Notice of Privacy Practices:
- Notice of Privacy Practices
- Acknowledgement of Receipt of Notice of Privacy Practices
- Notice/Acknowledgement of Privacy Practices (TOPA)
For more information about your health information privacy rights, click HERE.
MOLECULAR TESTING: ROADMAP TO CANCER
Most people agree that using a Google™ map or similar navigation device ensures that a traveler will reach their destination accurately and more quickly than without such guidance. Next Generation Genomic Sequencing (NGS) represents the current, state-of-the-art molecular test and works like a roadmap for physicians and their cancer patients.
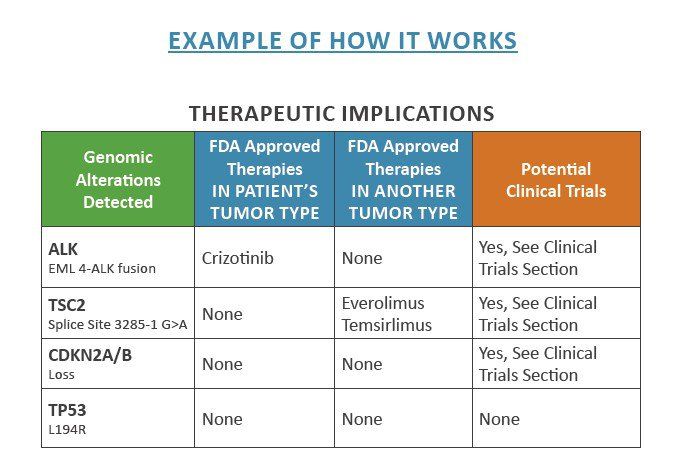
Molecular testing through NGS is routinely ordered for each patient at Mary Crowley that has a tissue sample of their tumor from an earlier surgery. The results identify the gene alterations/biomarkers that contribute to the patient’s cancer growth and act as a roadblock to traditional cancer treatments. Identification of gene mutations is valuable for the physician, but only with accurate interpretation and correlated therapies that address the molecular signature of the patient. With this information, a patient can be appropriately aligned to a matching therapy designed to impact that mutation or cancer signal (see below for an example of how this works in a Foundation One™ partial report).
All patient sequencing results are maintained in a dynamic Patient Molecular Registry at Mary Crowley and regularly assessed for a potential clinical trial match. Additionally, any patient may request Mary Crowley to conduct NGS of a portion of their surgical tumor in the event they may need a research option, if needed, after their initial cancer surgery and standard treatment.