Breakthrough in the Treatment of Locally Advanced or Metastatic KRASG12C & Non-Small Cell Lung Cancer
Monday, November 20, 2023
Dr. Minal Barve

In December 2022, the FDA approved the treatment of adult patients with KRASG12C mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) with adagrasib (Krazati), becoming the 20th drug approval at MCCR.
This breakthrough in the treatment of NSCLC was possible from the KRYSTAL-1 phase1-1b study of adagrasib which demonstrated promising results.
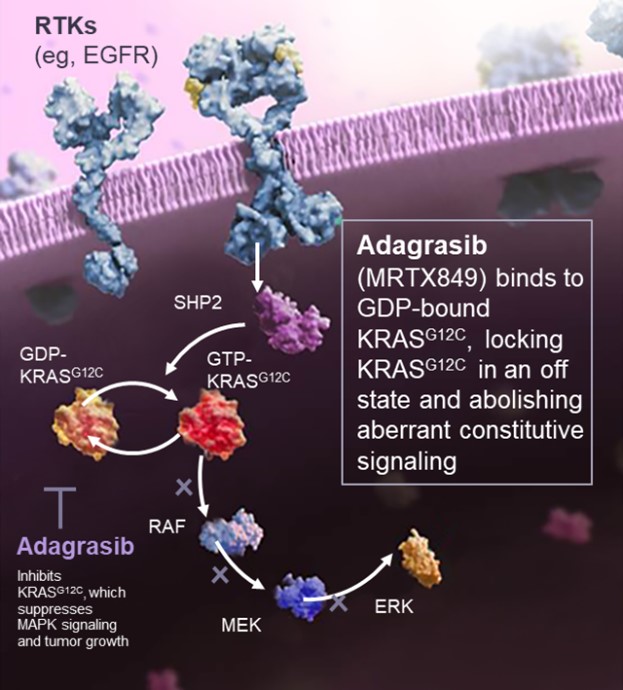
The KRAS protein is part of a cell signaling pathway which stimulates cell growth and proliferation. In cancers with the KRASG12C mutation, the KRAS protein is hyperactive, leading to uncontrolled cell growth. Adagrasib combats this by binding to the KRAS protein and inhibiting its activity, causing tumor cell shrinkage and death. Favorable properties of this molecule is its long half-life, central nervous system (CNS) penetration and dose dependence.
In the KRYSTAL-1 study, adagrasib was administered to NSCLC patients previously treated with platinum-based therapy and PD1/PDL1 therapy. Compared with baseline disease measurements, 42.9 percent had confirmed responses to adagrasib, with a median duration of response of 8.5 months, a median progression-free survival of 6.5 months, and a median overal survival of 12.6 months. adagrasib showed clinical efficacy with manageable toxicity with an acceptable adverse event profile.
Adagrasib is also being studied for its treatment of KRASG12C colorectal cancer, pancreatic cancer as well as other solid tumors. Recently, Mary Crowley along with Mirati Therapeutics published a manuscript on the use of adagrasib with or without Cetuximab (Erbitux), an injection-based chemotherapy that targets EGFR expressing tumor cells, in The New England Journal of Medicine.
While Adagrasib is not currently approved for its treatment of KRASG12C mutated colorectal cancer, Dr. Minal Barve, MD, Executive Medical Director at Mary Crowley, is hopeful the FDA will approve this application of adagrasib for colorectal cancer as well other solid tumors that have KRASG12C mutation.